Primary use
Type of medical device or related product
General vs. specific use (health condition)
Intended population age
Intended population sex
Level of technical knowledge
Capital
Reusable
Requirements
air conditioning / temperature control
electricity (mains)
lighting
WHO list of priority medical devices
Service delivery platforms / Healthcare levels
Healthcare unit
EMDN related code(s)
Z12020703
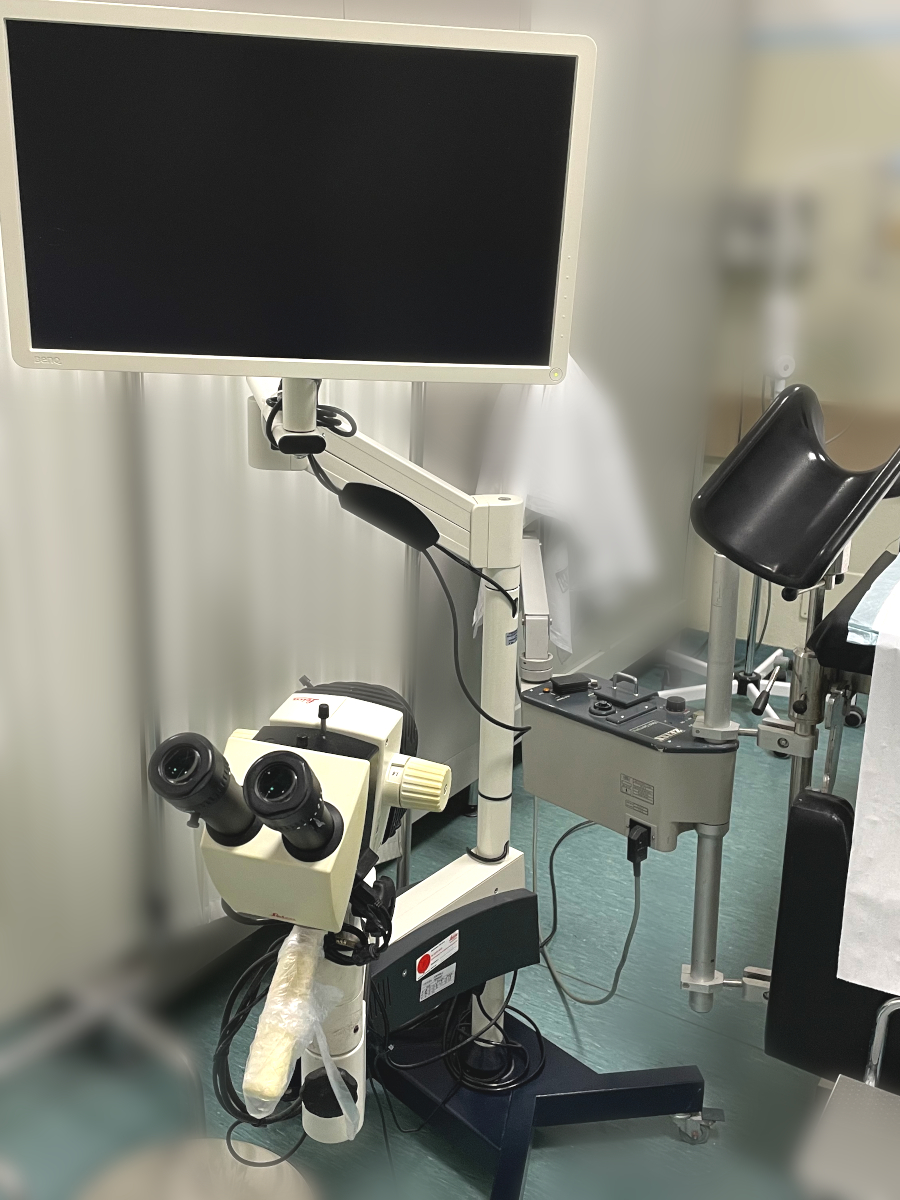
COLPOSCOPES
The code(s) and term(s) in this section were observed and retrieved from public databases and have not been validated by health regulatory authorities.
Please consult your regulatory agency and EMDN site:
https://webgate.ec.europa.eu/dyna2/emdn
GMDN related code(s)
10960
Colposcope (An electrically-powered instrument intended to be used for the visual examination and treatment of the female genitalia. It is placed directly at the opening of the vagina which is held open with an inserted speculum during colposcopy. The instrument has a light source and magnifying lenses that allow the physician to view the interior of the vagina, particularly the uterine cervix. This device is used to check for cervical cancer typically after an abnormal Papanicolaou test (PAP) or as a follow up procedure to view an abnormal area seen during an earlier gynaecological examination. During colposcopy, samples of tissue can be taken from the cervix for testing.)
The medical device term(s), code(s) and definition(s) in this section were retrieved from databases external to WHO.
As there might be more than one name, definition and “Nomenclature Code” related to the specific medical device, please consult
https://gmdnagency.org
GMDN ®. © GMDN Agency 2005-2024
WHO Technical specifications
WHO related resources
WHO resources on medical devices
Interagency list of priority medical devices for essential interventions for reproductive, maternal, newborn and child health
WHO list of priority medical devices for cancer management
WHO List of Priority Medical Devices for management of cardiovascular diseases and diabetes
WHO List of Priority medical devices list for the COVID-19 response and associated technical specifications
Package of eye care interventions
Trauma and Emergency Surgery Kit (TESK) 2019
WHO medical devices website
WHO priority medical devices website
WHO essential in vitro diagnostic website


