Primary use
Type of medical device or related product
General vs. specific use (health condition)
Intended population age
Intended population sex
Level of technical knowledge
Capital
Reusable
Requirements
electricity (batteries)
electricity (mains)
WHO list of priority medical devices
Service delivery platforms / Healthcare levels
Healthcare unit
EMDN related code(s)
Z1203020408
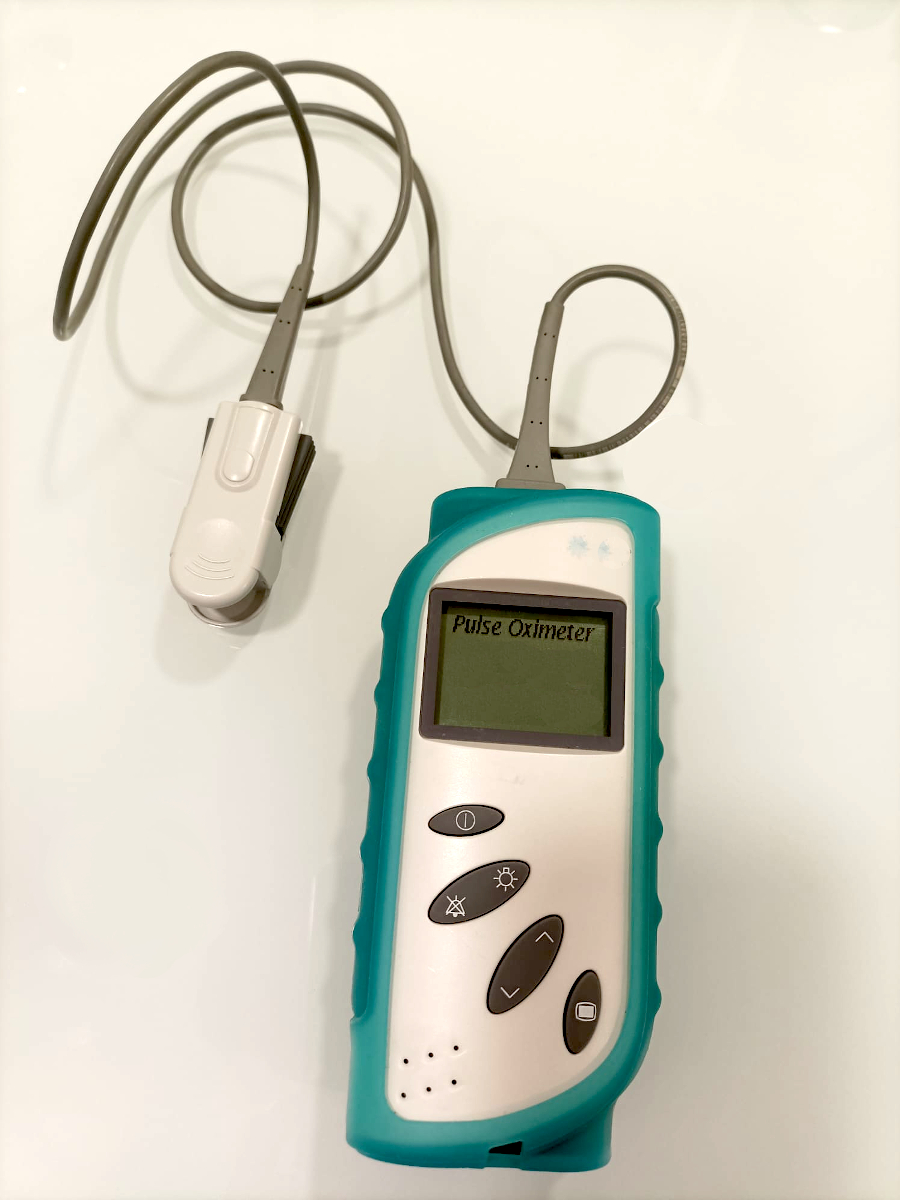
PULSE OXIMETERS
The code(s) and term(s) in this section were observed and retrieved from public databases and have not been validated by health regulatory authorities.
Please consult your regulatory agency and EMDN site:
https://webgate.ec.europa.eu/dyna2/emdn
GMDN related code(s)
45607
Pulse oximeter (An electrically-powered photoelectric device designed for the transcutaneous measurement and display of haemoglobin oxygen saturation (SpO2). The signals are produced by light-emitting diodes (LEDs) and received by a photodetector. The device displays the SpO2 values and may also measure/display pulse rate. It is typically applied to the fingertip or around the wrist, may be single-component (with built-in probe) or multi-component (includes external probe), and may in addition wirelessly transmit measurements to a receiving location (e.g., central station, bedside monitor); it is intended to be operated by laypersons and healthcare providers.)
The medical device term(s), code(s) and definition(s) in this section were retrieved from databases external to WHO.
As there might be more than one name, definition and “Nomenclature Code” related to the specific medical device, please consult
https://gmdnagency.org
GMDN ®. © GMDN Agency 2005-2024
WHO Technical specifications
WHO related resources
http://apps.who.int/iris/bitstream/10665/44185/1/9789241598552_eng.pdf
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management
https://www.who.int/publications/i/item/basic-emergency-care-approach-to-the-acutely-ill-and-injured
https://www.who.int/emergencycare/systems/en/
https://www.who.int/publications/i/item/guidelines-for-essential-trauma-care
Training materials
WHO resources on medical devices
Interagency list of priority medical devices for essential interventions for reproductive, maternal, newborn and child health
WHO list of priority medical devices for cancer management
WHO List of Priority Medical Devices for management of cardiovascular diseases and diabetes
WHO List of Priority medical devices list for the COVID-19 response and associated technical specifications
Package of eye care interventions
Trauma and Emergency Surgery Kit (TESK) 2019
WHO medical devices website
WHO priority medical devices website
WHO essential in vitro diagnostic website


