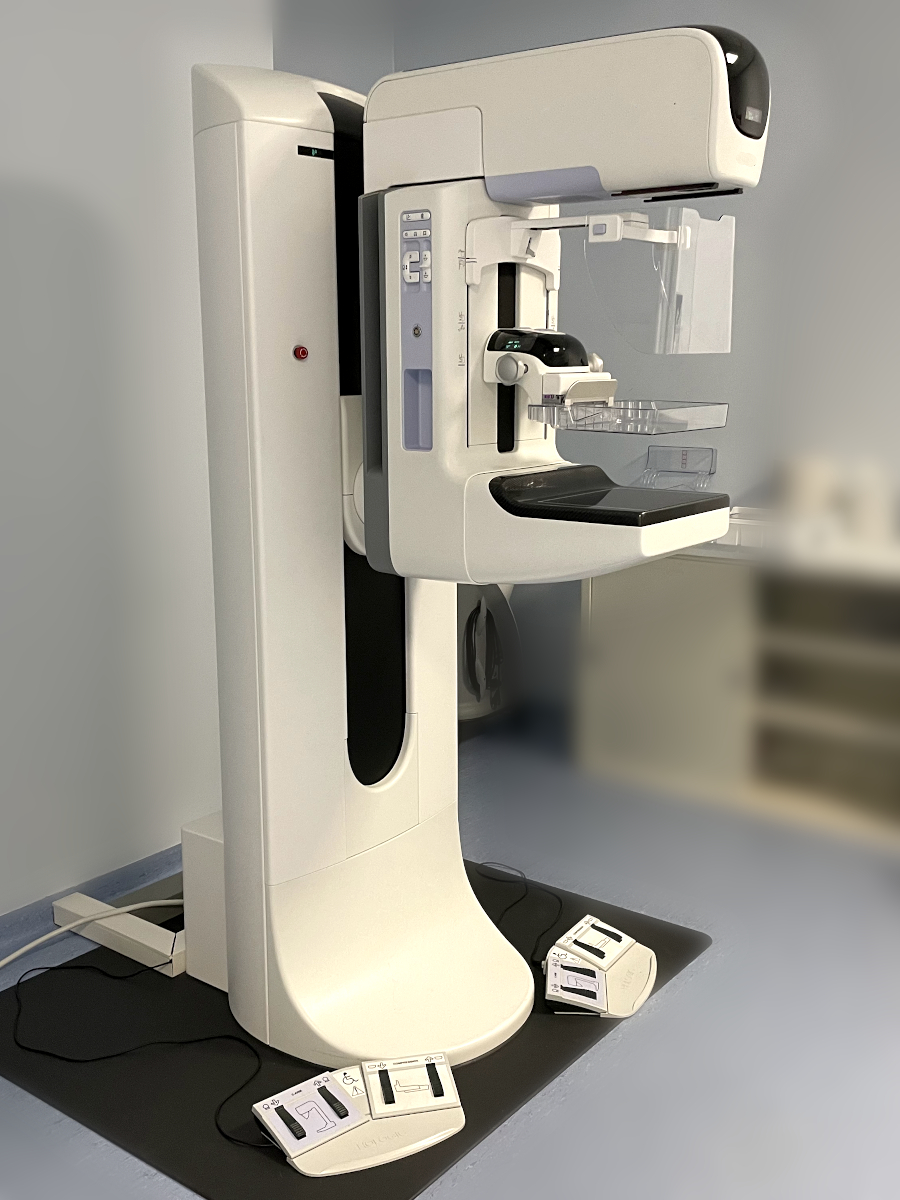
Alternative names
Mammograph
Primary use
Type of medical device or related product
General vs. specific use (health condition)
Intended population age
Intended population sex
Level of technical knowledge
Capital
Reusable
Requirements
air conditioning / temperature control
communication system
electricity (mains)
emergency power supply
Fire detection and protection
HIS/LMIS/RIS/PACS
lighting
pre-installation preparation / mechanical guides
radiation protection
reinforced floor or ceiling
WHO list of priority medical devices
Service delivery platforms / Healthcare levels
Healthcare unit
EMDN related code(s)
Z110302
MAMMOGRAPHY SYSTEMS
The code(s) and term(s) in this section were observed and retrieved from public databases and have not been validated by health regulatory authorities.
Please consult your regulatory agency and EMDN site:
https://webgate.ec.europa.eu/dyna2/emdn
GMDN related code(s)
37672
Stationary mammographic x-ray system, digital (A stationary assembly of devices designed to generate x-ray images of the breast using digital techniques for image capture and display. It is designed specifically to compress the breast during imaging and is intended to visually evaluate the anatomy and function of blood and lymphatic vessels within the breast. Often referred to as a digital mammography system (DMS) it is typically used for breast cancer screening or during biopsy procedures (e.g., placement of biopsy markers, stereotactic biopsy). It is designed to capture two-dimensional (2-D) x-ray images, however may include software intended to process multiple images to create a three-dimensional (3-D) image/model (tomosynthesis).)
The medical device term(s), code(s) and definition(s) in this section were retrieved from databases external to WHO.
As there might be more than one name, definition and “Nomenclature Code” related to the specific medical device, please consult
https://gmdnagency.org
GMDN ®. © GMDN Agency 2005-2024
WHO related resources
WHO resources on medical devices
Interagency list of priority medical devices for essential interventions for reproductive, maternal, newborn and child health
WHO list of priority medical devices for cancer management
WHO List of Priority Medical Devices for management of cardiovascular diseases and diabetes
WHO List of Priority medical devices list for the COVID-19 response and associated technical specifications
Package of eye care interventions
Trauma and Emergency Surgery Kit (TESK) 2019
WHO medical devices website
WHO priority medical devices website
WHO essential in vitro diagnostic website


